Singapore studying effectiveness and safety of Moderna, Pfizer-BioNTech’s COVID-19 vaccines for children 6 months and older
Singapore studying effectiveness and safety of Moderna, Pfizer-BioNTech’s COVID-19 vaccines for children 6 months and older
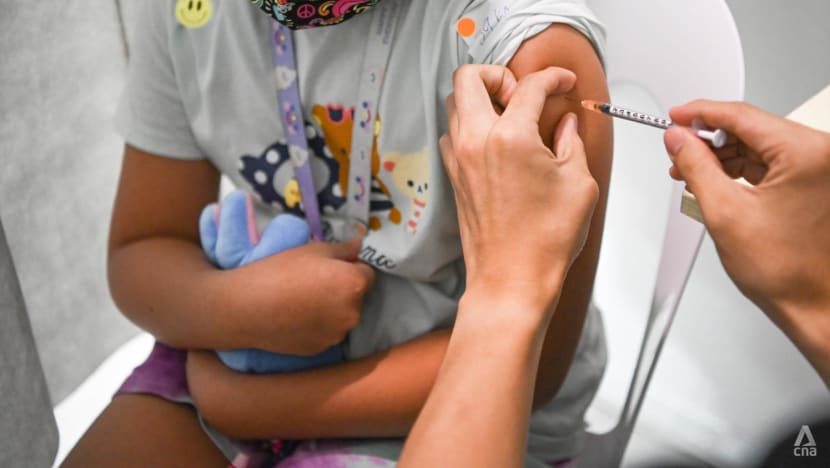
The Ministry of Health (MOH) and the Expert Committee on COVID-19 Vaccination are currently studying the effectiveness and safety of Moderna's and Pfizer-BioNTech’s vaccines in young children.
สล็อตxo168 Notification of the rules and rules of the website is very necessary for bettors to use online web slot services. Read and understand to match online slots
The study will look at the use of Moderna's COVID-19 vaccine for children aged six months to five years, and Pfizer-BioNTech's vaccine for those aged six months to four years.
“We are engaging the respective vaccine manufacturers to obtain the relevant information to facilitate a risk-benefit evaluation of the use of these vaccines in this age group,” said MOH on Monday (Jun 20) in response to media queries.
The US Centers for Disease Control and Prevention on Saturday recommended COVID-19 vaccines for children as young as six months, allowing a nationwide rollout to start next week. The CDC's move came after a panel of advisers voted to recommend COVID-19 vaccines for those children.
The US Food and Drug Administration on Friday authorised Moderna's shot for children aged six months to five years, and Pfizer-BioNTech's vaccine for children aged six months to four years. Pfizer-BioNTech's vaccine is already authorised for children over the age of five in the US.
The Health Ministry began its vaccination exercise for children aged five to 11 on Dec 27 last year.
โพสตอบ
* ต้องล็อกอินก่อนครับ ถึงสามารถเโพสตอบได้
